Put Tellurium in center and arrange fluorine atoms on the sidesArrange electrons until fluorine atoms get 8 electrons Tellurium gets 10 electrons which is an expanded octet. Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the elements symbol.
Alternatively a dot method can be used to draw the lewis structure.
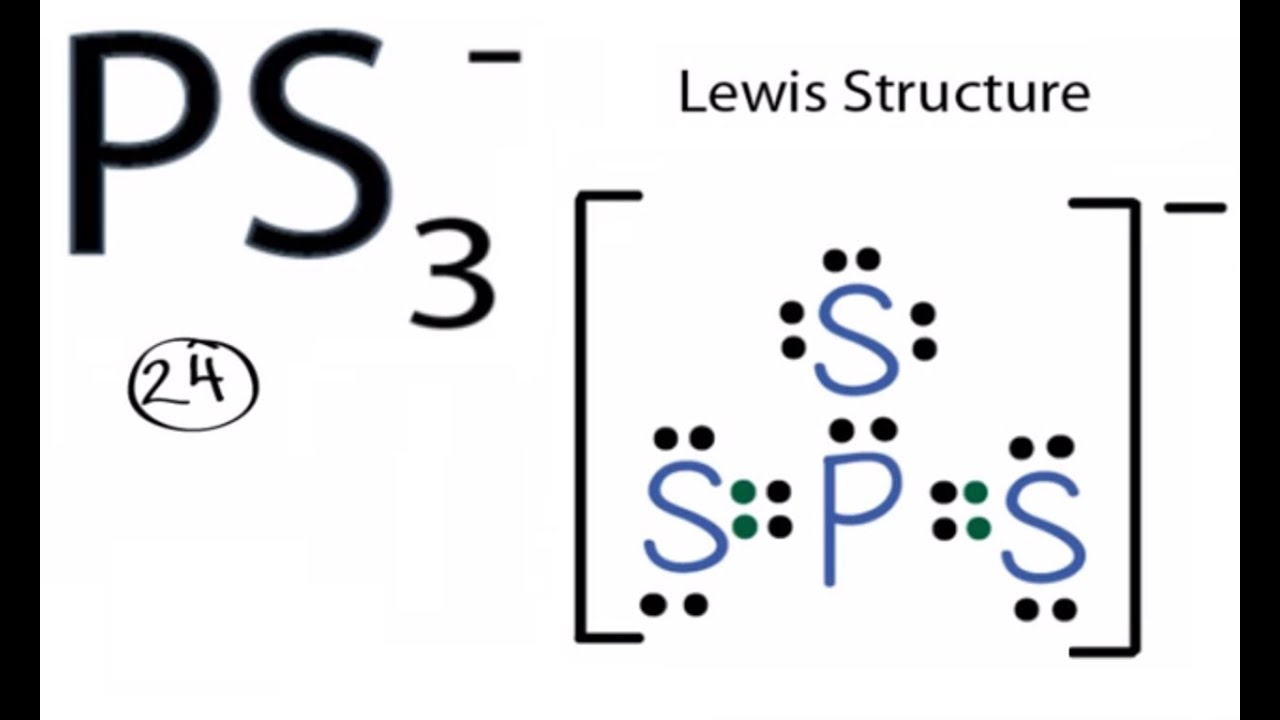
Genuine lewis dot diagram and the description. Lewis structures depict the bonds between atoms of a molecule as well as any unbonded electron pairs. Calculate the total valence electrons in the molecule. The Lewis structure was named after Gilbert N.
Lewis Structure for NO 2-Nitrite ion. Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. Lewis who described them in a 1916 article titled The Atom and the Molecule.
By circling the electrons around each atom we can now see that the O and C atoms have octets while each H atom has two electrons. Lewis Structuresdoc BRIEF SUMMARY OF LEWIS STRUCTURES. 21082019 In the case of CH 2 O the O and C atoms share two pairs of electrons with the following Lewis electron dot diagram as a result.
He described what he called the cubical atom because a cube has 8 corners to represent the outer valence shell electrons which can be shared to create a bond. One dot is place on each side first and when all four positions are filled the remaining dots are paired with one of the first set of dots with a. 23072020 The Lewis Dot Structure is a visual which represents the outermost shell of electrons also known as valence electrons and possible covalent bonds within an atom or molecule.
Each valence shell is full so this is an acceptable Lewis electron dot diagram. Lewis structures also known as Lewis dot formulas Lewis dot structures electron dot structures or Lewis electron dot structures are diagrams that show the bonding between atoms of a molecule as well as the lone pairs of electrons that may exist in the molecule. Lewis structure of NO 2-ion is drawn in this tutorial.
These valence electrons are negatively charged and are attracted to the positively charged nucleus made up of neutrons and protons. Lewis suggested that a chemical bond involved sharing of electrons. The Lewis structure was named after Gilbert N.
A beryllium atom with two valence electrons would have the electron dot diagram below. The Lewis structure of a positive ion cation is positioned adjacent to the Lewis structure of a negative ion anion. When examining chemical bonding it is necessary to keep track of the valence electrons of each atom.
The valence electrons are written as dots surrounding the symbol for the element. A Lewis electron dot diagram or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The resultant difference in charge between the ions so formed is responsible for the bonding forces in ionic materials.
Ionic bonds are the result of the. Lewis dot structure of eqSiO_44 - eq contains Si which is bonded to 4 oxygen atoms. Lewis dot structures are commonly referred to as electron dot structures or Lewis structures.
Now we are going to learn how to draw this lewis structure. Or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. These dots are arranged to the right and left and above and below the symbol.
In 1916 ten years before the Schrodinger wave equation G. Transfer giving taking of one or more electrons from one atom or groups of atoms to another atom or group. This was his octet rule.
08122019 Lewis structures also known as electron dot structures are named after Gilbert N. The number of dots equals the number of valence electrons in the atom. Total valence electrons of nitrogen and oxygen atoms and negative charge also should be considered in the drawing of NO 2-lewis structure.
Lewis who introduced it in his 1916 article The Atom and the Molecule. Each oxygen atom is having a negative charge and the valence electrons of. The Lewis Structure electron dot diagram of each ion is used to construct the Lewis Structure electron dot diagram for the ionic compound.
A step-by-step explanation of how to draw the O2 Lewis Dot Structure Oxygen Gas Diatomic OxygenFor the O2 structure use the periodic table to find the t. The number of dots equals the number of valence electrons in the atom. They also display the total number of lone pairs present in each of the atoms that constitute the molecule.
Lewis who introduced it in his 1916 article The Atom and the Molecule4 They are similar to electron dot diagrams in that the valence electrons in lone pairs are represented as dots but they also contain lines to represent shared pairs in a chemical bond single double triple etc Formal charge. Lewis dot diagrams for elements are a handy way of picturing valence electrons and especially what electrons are available to be shared in covalent bonds. A Lewis structure can be drawn for any covalently bonded molecule as well as coordination compounds.
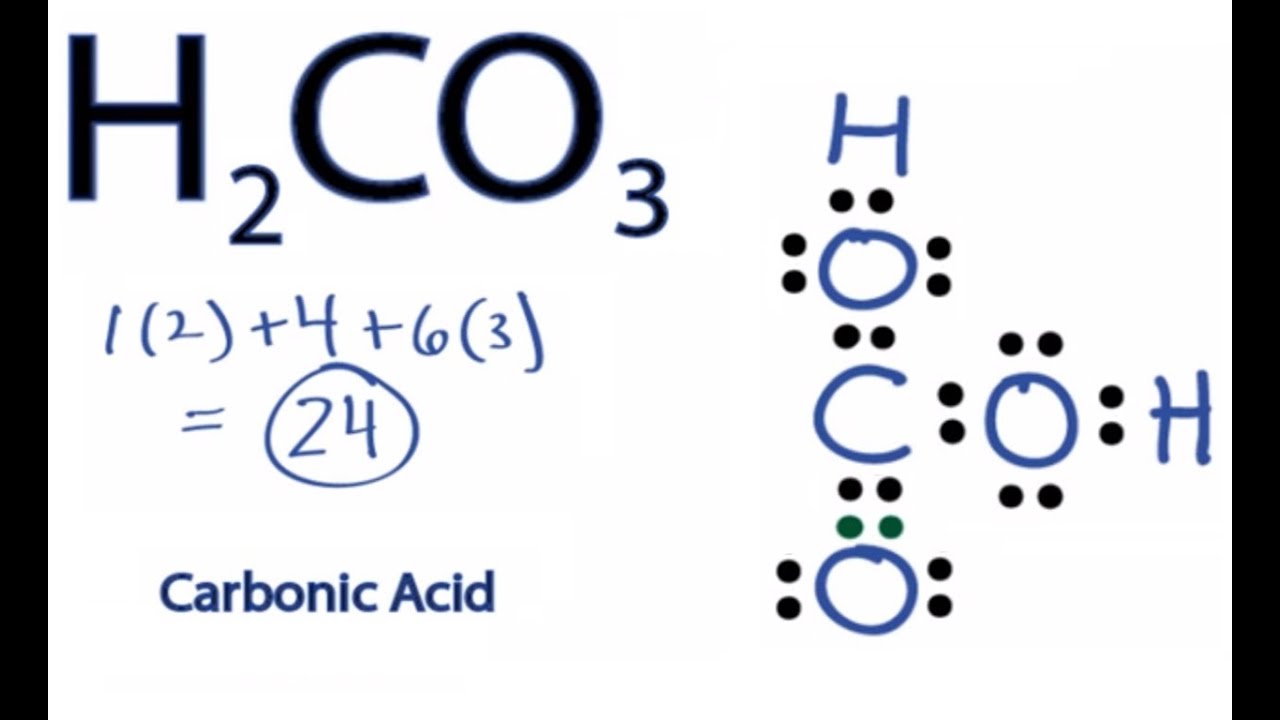 Diagram Lewis Diagram H2co3 Full Version Hd Quality Diagram H2co3 Imdiagram Arsae It
Diagram Lewis Diagram H2co3 Full Version Hd Quality Diagram H2co3 Imdiagram Arsae It
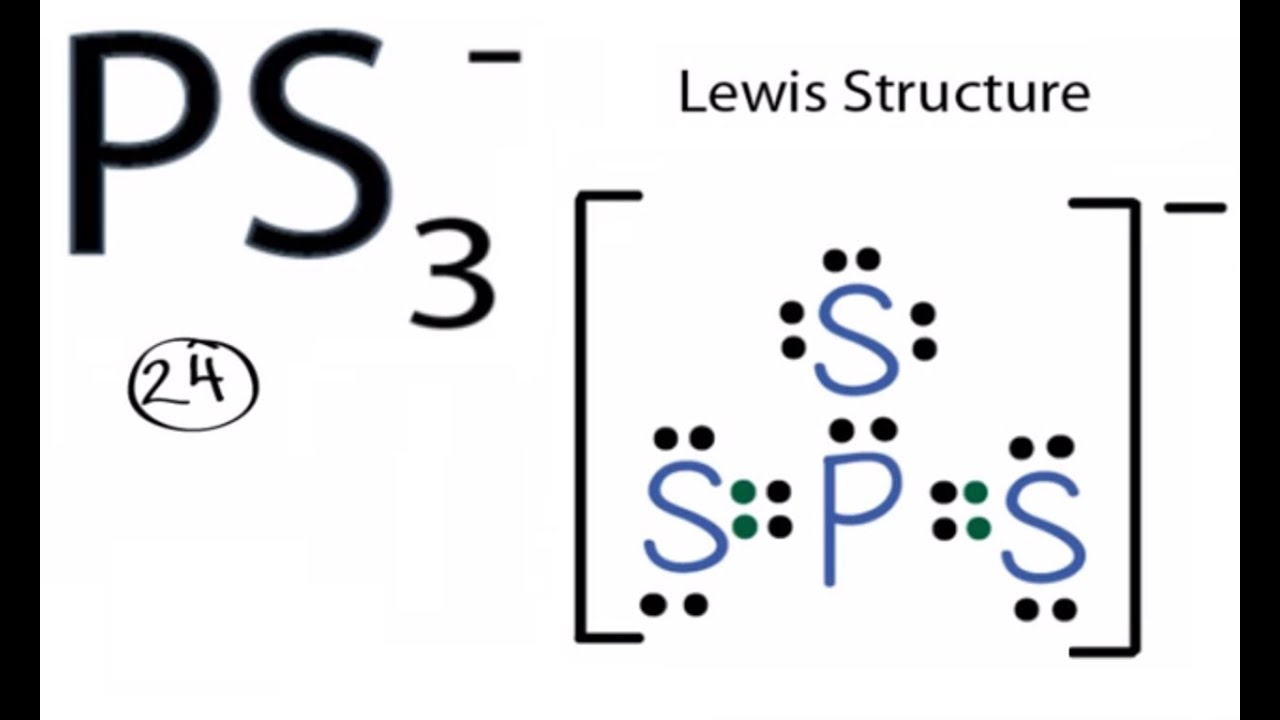 Diagram Element Lewis Diagram Full Version Hd Quality Lewis Diagram Tvdiagram Premioraffaello It
Diagram Element Lewis Diagram Full Version Hd Quality Lewis Diagram Tvdiagram Premioraffaello It
What Is The Dot Structure Of Calcium Carbide Quora












0 Response to "[6+] Genuine Lewis Dot Diagram And The Description"
Post a Comment